Reimbursement
Device Reimbursement Approval
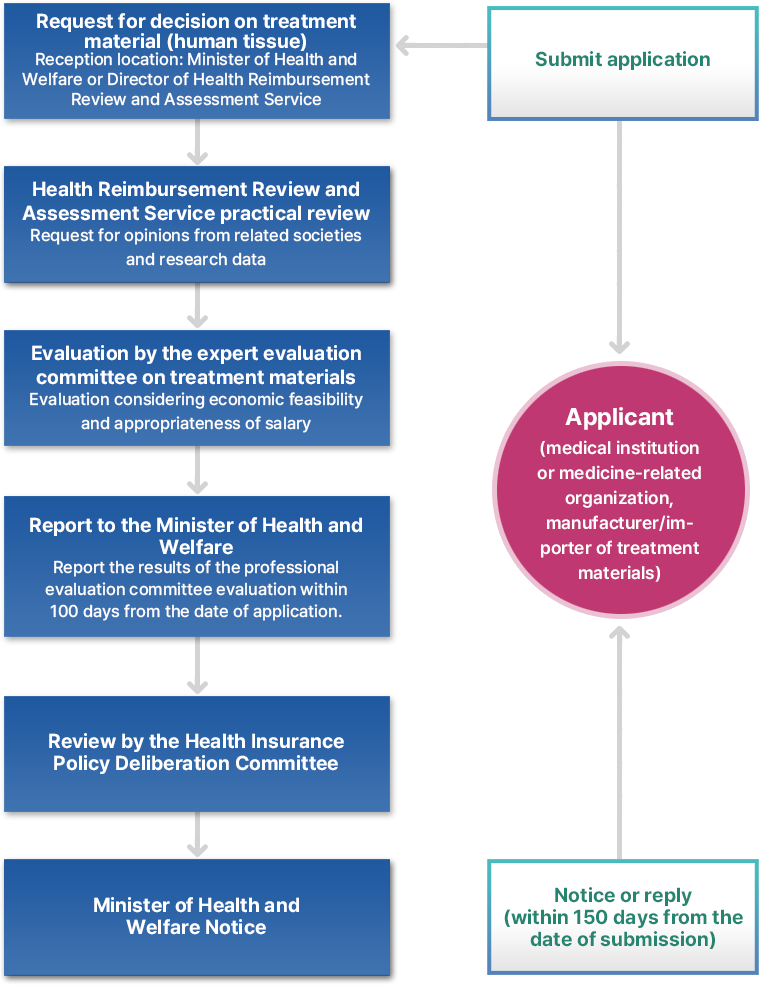
Treatment material decision process
Among medical devices that have been notified, certified, and approved by the Ministry of Food and Drug Safety (MFDS), consumable materials are called “therapeutic materials.”
In the current health Reimbursement benefit system, it is divided into benefit and non-benefit.
It is managed by listing it in the “Treatment materials benefit, non-benefit list and benefit ceiling amount table”.
The application process for listing the above treatment materials is called “determination of treatment materials” and is a procedure to apply to the Health Reimbursement Review and Assessment Service within 30 days of reporting, certification, and approval to review the adequacy of benefits by reviewing the safety, effectiveness, and economic feasibility of the treatment materials. This is called “application for decision on treatment materials.”