Reimbursement
Eligibility for the Medical Care Service
What is the rule of confirmation application system of Medical care covered benefit/non-covered benefit target ?
This refers to those eligible for medical care covered benefits pursuant to Article 8 of the National Health Insurance Medical Care Benefits Standards Rules or those subject to non-reimbursement pursuant to Article 9 of the same Rules.
Application for Verification of Eligibility for Covered and Non-covered Benefits in Medical Care Services
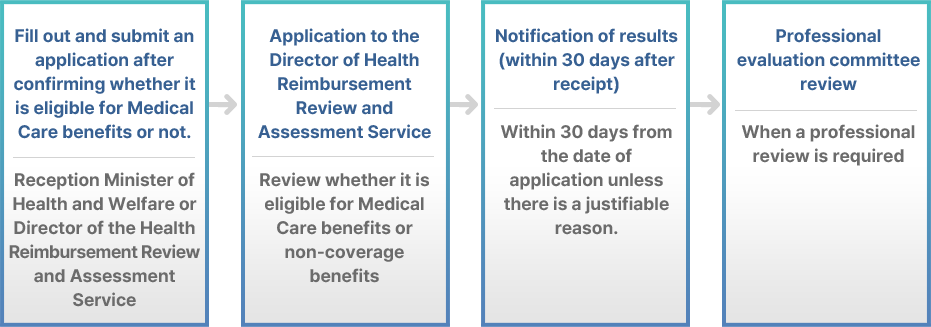
· The application system for confirmation of eligibility for health care benefits or non-benefit refers to the application for new medical technology evaluation under Article 53 of the 「Medical Service Act」 and the application for deferral of new medical technology evaluation under Article 3 of the 「Rules on New Medical Technology Evaluation」. This is a system to apply to the Minister of Health and Welfare for confirmation of whether or not an act is eligible for medical care covered benefits or non-covered benefits for an act whose the technology is unclear.
· When an application for confirmation of eligibility for medical care covered benefits or non-covered benefits is submitted, if a professional review is required to confirm eligibility for them, after review by the professional evaluation committee according to Article 11 (7), 30 days from the date of application unless there is a justifiable reason. You will be notified of the results within 30 days. However, if the confirmation based on previous decisions is difficult and in-depth review is necessary, the notification period may be extended once within the range of 30 days.
Appeal for Review of Determination on Eligibility for Covered and Non-covered Benefits in Medical Care Services
An appeal must be submitted, along with the necessary documents, to the Director of the Health Insurance Review and Assessment Service within 30 days from the date of receiving the notification regarding the determination of eligibility for covered and non-covered benefits in medical care services.
※ Related evidence - Article 9-2 of the Rules on Standards for National Health Insurance Medical Care Benefits
Integrated operating system
· If you submit the ‘Application for Medical Device Manufacturing (Import) Permission and Integrated Operation of New Medical Technology Evaluation’ and the ‘Application for Confirmation of Eligibility for Nursing Benefits/Non-Benefits’ to the Ministry of Food and Drug Safety, you will receive one result that reflects all three results.
Integrated operation target
The purpose of use of the medical device for which permission is sought must be the same as the purpose of use of the medical technology stated in the application under Article 3, Paragraph 1, Item 1 of the Regulations on Integrated Operation of Medical Device Permission, New Medical Technology Evaluation, etc.
※ Related evidence
- Article 9-2, Paragraphs 2 and 3 of the Rules on Standards for National Health Insurance Medical Care Benefits
- Regulations on integrated operation such as medical device approval and new medical technology evaluation (Ministry of Health and Welfare Notice No. 2017-211 (2017.11.29.))
Processing Procedure